Genevron, the ALSA and Me
 My least favorite blog post subject is ALS. This is true when it comes to ALS in general, and it is even more true when it comes to my ALS in particular. I’d rather doodle about birds, crossword puzzles, music, books, complete nonsense and even the Timberwolves than about ALS. But this is a very important time for People with ALS (PALS) in general, and since my recently-dropped enigmatic references to my ALS on Facebook have generated questions and concern, I might as well address my particular situation as well.
My least favorite blog post subject is ALS. This is true when it comes to ALS in general, and it is even more true when it comes to my ALS in particular. I’d rather doodle about birds, crossword puzzles, music, books, complete nonsense and even the Timberwolves than about ALS. But this is a very important time for People with ALS (PALS) in general, and since my recently-dropped enigmatic references to my ALS on Facebook have generated questions and concern, I might as well address my particular situation as well.
Photo above taken from the March 25 rally in Washington DC in support of accelerated approval for GM604.
First the important stuff.
Genervon, the FDA and the ALSA
More than a month ago on Facebook I posted links to a petition site and encouraged friends and family to support the small pharmaceutical firm Genervon’s proposal to the FDA asking to have the Accelerated Approval Program applied to its experimental drug, GM604.
You and many many others have come through in a big way. More than a half million signatures have been gathered at change.org in support of Genervon. A decision by the FDA is expected any day now. “Sometime in March” is what we were told to expect.
The FDA is taking its time. Perhaps that is a good sign. Still, I am not optimistic this ruling will go the way I hope it does. I and many others have been very disappointed that the ALSA has declined to support the large majority of its constituency that has come out in favor of Genervon in this matter. We want the opportunity to decide for ourselves whether or not we are willing to assume the risks of taking this medication now after its successful phase II trial and before the three years or more that a complete, traditional, phase III trial and analysis has been completed.
I do not need to explain everything I know about this experimental drug and the fight to get it approved for a special “phase IV confirmatory trial” that would allow PALS otherwise ineligible for medical trials to gain access to this compound. Others have done this work for me. To read more about this, click on any of these links:
Stephen Finger’s “Case for Accelerated Approval”
Early promise notwithstanding, it is a long shot that this drug will prove to be of much help to those of us with ALS. It will be wildly successful if it can slow progression to any significant extent. No one is under the illusion that it might “cure” ALS. Still, it is potentially a step in the right direction. It already too late to do me much good, but future generations of PALS could benefit enormously.
As much as anything else, accelerated approval would signal a change in the FDA’s approach to drug development for ALS. That in itself would be a big step. With copious historical control data from previous trials available, there is no reason not to allow what the FDA calls a “phase IV confirmatory trial.”[1]
 Shame, shame on the ALSA for being so unsupportive. I may lay out more of my reasons for my feelings in another post. Here I will only say that I believe ALSA’s CEO Barbara Newhouse has either been dishonest with the ALS community or she is incompetent. The ALSA no longer represents me and I will donate elsewhere (ALS TDI) and encourage friends and family to do the same.[2]
Shame, shame on the ALSA for being so unsupportive. I may lay out more of my reasons for my feelings in another post. Here I will only say that I believe ALSA’s CEO Barbara Newhouse has either been dishonest with the ALS community or she is incompetent. The ALSA no longer represents me and I will donate elsewhere (ALS TDI) and encourage friends and family to do the same.[2]
Me, an update (warning: self-pity)
Yes, I am getting worse. Not unexpectedly. The disease marches on, but more slowly than I could have hoped five years ago when I was diagnosed with “probable ALS,” and four years, one month and 16 days since it was made official. (That is 1,505 days since my definitive diagnosis. Not that I am keeping track of the time or anything like that.)
So what is changing?
I am fast losing the ability to do some of my most favorite things: walk, talk and scratch my nose. I can, however, still scratch my ass. But only the right cheek.
Using my computer will become very difficult over the next year or less. Right now I move a wireless mouse with my right hand. I press specially-adapted mouse buttons with my right foot. I dictate 98 percent of what I write using Dragon NaturallySpeaking. I use an on-screen keyboard to type the other two percent.
It’s a clunky system, but it is functional. The end of it is coming. Dragon misinterprets my speech in more interesting ways every day. My mouse movements are getting jerkyier and herkier and the small movements required tire me. My attempts to sample eye-gaze technology at the VA have been uninspiring at best. I hope I will never be dependent upon it. But there is a really only one way to avoid that fate and I am not eager for that solution just yet.
 More immediately, my loss of mobility is leading to some distinct unpleasantness. Twice in the past month I have fallen painfully. I’m not looking forward to the next fall, but the only way to ensure it doesn’t happen is to stay seated. Forever. I’m not quite ready—or smart enough—to accept this.
More immediately, my loss of mobility is leading to some distinct unpleasantness. Twice in the past month I have fallen painfully. I’m not looking forward to the next fall, but the only way to ensure it doesn’t happen is to stay seated. Forever. I’m not quite ready—or smart enough—to accept this.
One day last week, an urgent need expressed itself and I was able to shuffle to the toilet and, after an epic 15-minute struggle, to get my pants (read: pajama bottoms) down to my ankles. A big win!
I could not, however, get myself off of the toilet. So I waited 45 minutes for my personal care attendant (PCA) Julie to arrive for one of her regularly-scheduled visits. (Right now she is here twice a week to feed me lunch and do some range-of-motion exercises. This allows Joann to provide office hours for her students and to attend meetings on Tuesdays and Fridays.)
Julie and I got better acquainted. She was wonderfully compassionate and helpful. Got me to my feet, wheeled me to a position where she could restore my pants and at least some of my dignity.
Once I accept that I can no longer stand, this kind of assistance will become routine. It will become the new normal. I will need to have a PCA with me whenever I would otherwise be alone.
A couple of days ago I had my worst choking incident to date. Scary and painful. It is getting harder to coordinate all of the complicated physical processes involved in swallowing. Both my nutritionist and speech pathologist have explained to me in detail how all of that works. It is a wonder we don’t all choke to death on a weekly basis.
So, fewer and fewer things I can safely eat and, down the road, a feeding tube. I hope I never get there but, again, the only way I won’t is if I “leave the party” (to use my favorite euphemism for what is technically known as “taking a dirt nap”).
Still, I know I am more fortunate than many.
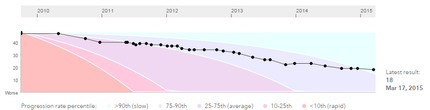
Here’s a look at the progression I am making on the ALS Functional Rating Scale (ALS/FRS). Click on the image above to enlarge it. You will see that I am now categorized with the most slowly-progressing percentile of PALS. And I know the statistics do not lie. It seems that almost all of the PALS in my “generation"—those diagnosed within a year or so of me—are gone. I can see the faces and remember the names of so many I knew so briefly: Rob, Elizabeth, Al, Anne, Tom, Renee, Bob, John, Bruce and so many more. It is a list that will only continue to grow. Here’s hoping a change to the status quo is coming.
| DATE | TITLE |
| 02/28/2019 | Unhappy Anniversary |
| 05/12/2017 | The Eyes Have It |
| 08/04/2016 | YFALS National Corntoss Challenge |
| 07/18/2016 | Scopolamine Blues |
| 03/06/2016 | Dragon Me Down |
or see the Complete ALS Bites
—
Notes
- The ALSA, along with a few other ALS-focused organizations including the MDA, issued a press release outlining their reasons for not supporting Genervon’s petition.
I do not disagree that research into this drug is very preliminary. We have seen hopeful results in early studies before. There is no guarantee or even a likelihood that GM604 will prove to be effective. But the concerns expressed by the ALSA about its safety are disingenuous. There is very little reason to believe that this compound will be harmful to PALS. Even if there were a significant risk—if there were less known about it than there is—it is a risk many PALS are willing to accept. The ALSA brings up an old lithium trial. A lithium trial that was shortened significantly because large numbers of ALS patients were able to get the drug outside of a trial and reported their results in forums such as PatientsLikeMe.com. This “open” trial was very effective in showing that lithium did not work and in fact performed negatively. Without input from the patients outside of a formal trial, research into the drug would have dragged on longer than it did.
Granting Genervon’s petition will be the fastest way to determine whether or not this drug can be of use.
See Eric Valor’s blog for the best information regarding this issue. [^]
- I accuse Barbara Newhouse of dishonesty based on an “open letter” she posted on Facebook. Newhouse wrote that the ALSA had “reached out” to Genervon but had not heard back. Several PALS asked Genervon directly about this. Genervon claims that they first reached out to the ALSA and continued to solicit their help with disappointing results. I don’t believe there is any reason for Genervon to lie about this. I can’t find this information again, but it was at one time on the ALSA’s Facebook page and/or the GM604 Breakthrough ALS treatment Facebook page I referenced above. [^]